IP-001
A novel cationic immune stimulant
The first asset from Immunophotonics’ broad intellectual property portfolio.
IP-001 Oncology
Transforming tumor ablations into a systemic immunotherapy
- May enhance local treatment outcomes that are currently being performed every single day.
- Our unique MOA drives the transformation of a routine ablation or radiation treatment from a local targeted therapy to a systemically active immunotherapy.
- The generation of a de novo T-cell response and reinvigorated T cells provide the potential to enhance downstream traditional immunotherapies.
- Clear opportunity to treat a wide variety of solid tumors, with early findings demonstrating a clinical effect.
Infectious Disease
Supercharging vaccines to enhance long-term viral protection
- The addition of our proprietary polymer and infectious disease vaccines has led to a potent immune response to immediately combat infection.
- The long-lasting B-cell response may support improved immunity over conventional vaccines alone.
- The combination can be applied to conventional vaccine approaches as well as novel intranasal applications.
Expansion Opportunities
Our foundational intellectual property and know-how supports significant expansion opportunities in this untapped field of medicinal polymer applications.
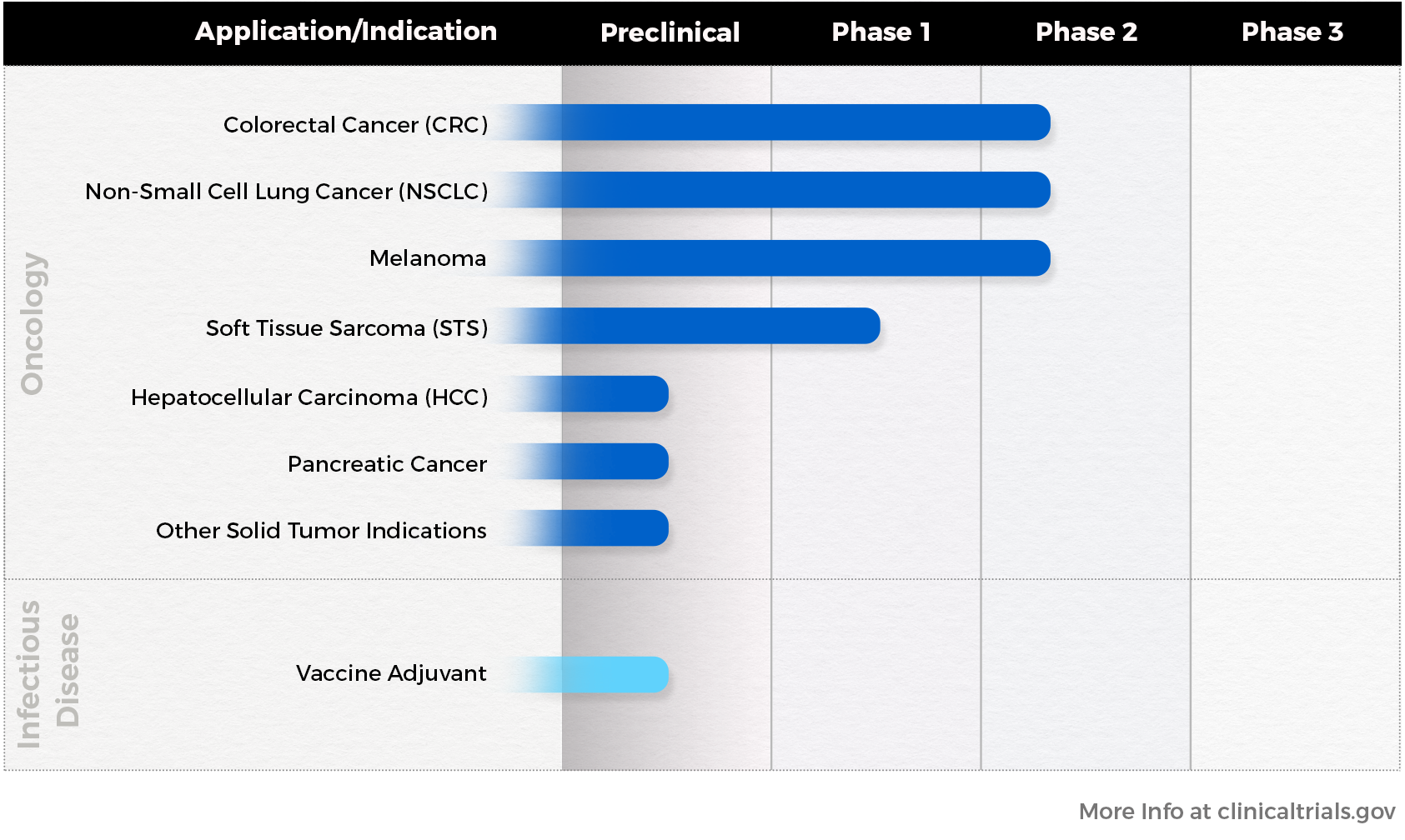
Colorectal Cancer (CRC)
The worldwide incidence of new colorectal cancer cases was 1,931,590 in 2020, with 935,173 patients succumbing to the disease that year. A large proportion of these cases will metastasize to and be ablated at the liver. It is estimated that by 2026 there will be over 230,000 CRC ablations and SBRT treatments performed in the U.S. and Europe, rendering the addressable market in this area enormous even before adjusting for a potential influx in demand for these procedures that would presumably result if immunotherapies such as IP-001 are found to make them more effective.
Non-Small Cell Lung Cancer (NSCLC)
Worldwide, approximately 2.2 million patients were diagnosed with lung cancers and 1.8 million patients died from the disease in 2020. The overwhelming majority of cases are non-small cell lung cancer (NSCLC) at 84%, with the remainder mostly consisting of small cell lung cancer at 13%. By 2026, it is estimated that the combined number of ablation and SBRT radiation treatments will stand at over 210,000 per year in the U.S. and Europe, with each of these having the potential to be enhanced in their efficacy by IP-001.
Melanoma
According to the International Agency for Research on Cancer, approximately 325,000 patients across the globe were diagnosed with melanoma of the skin in 2020. Although melanoma only accounts for about 1% of skin cancers, it is the source of a large majority of skin cancer deaths. While not as commonly ablated as some indications, many melanoma receive photothermal or other ablation treatments. Across the U.S. and Europe, it is estimated that a minimum of 32,000 phase III and phase IV melanoma patients are currently good candidates for ablation combined with IP-001.
Soft Tissue Sarcoma (STS)
In 2021, about 13,500 patients will be diagnosed with STS and 5,350 will die from STS in the U.S alone, with a majority of these patients being men. The most common types of sarcomas include undifferentiated pleomorphic sarcoma, liposarcoma, and leiomyosarcoma. In the U.S. and European markets, nearly 18,000 STS patients are estimated to be eligible for ablation combined, all of which could be combined with IP-001 and other immunotherapies.
Hepatocellular Carcinoma (HCC)
In 2020, an estimated 905,677 patients were diagnosed with liver cancers and 830,180 patients died from these cancers in 2021. The reported incidence of these cancers has more than tripled since 1980, with HCC by far the most common type of liver cancer, comprising about 75%. A majority of these patients are not eligible for surgery, making them prime candidates for ablation. In the U.S. and Europe, and in addition to other cancers ablated at the liver, over 22,000 HCC ablations will be performed by 2026. Each of these procedures presents an opportunity for use of IP-001, and if ablations prove more effective through combination with an immunoadjuvant such as IP-001, the number of HCC ablation procedures will likely reach new heights.
Pancreatic Cancer
The American Cancer Society estimates that 62,200 patients will be diagnosed nearly 50,000 will die from pancreatic in 2022 just within the U.S., accounting for about 3% of all cancers but about 7% of all cancer deaths. Pancreatic cancer most frequently metastasizes to the liver, which is also a common location for ablations to be performed. For these patients, the overall five-year survival metric currently stands at just 11% across all stages combined. Therefore, new treatments – such as those intended to boost the efficacy of ablating pancreatic cancer metastases in the liver – are sorely needed for this indication.
Vaccine Adjuvant
As demonstrated by the rapid emergence and spread of SARS-CoV-2, the virus causing COVID-19, novel infectious diseases and their variants present an ever-present threat to public health. Immunophotonics is studying the potential for IP-001 and its derivatives to act as an immunoadjuvant to boost vaccines and other treatments in the fight against COVID-19 and other infectious diseases.





