Lu Alleruzzo co-founder and CEO of Immunophotonics attended the NYSE 9th Annual 2022 IPO Summit. Very inspiring to hear from senior executives and advisors from some high-profile IPO’s and SPACs and how companies are entering the public market. 2022 is proving to be very exciting for Immunophotonics.
Category: Latest News
2022 St. Louis Dealmaker of the Year
The continued success of Immunophotonics is driven by the dedication and hard work of our team. Immunophotonics was one of 11 dealmakers honored on March 4th at the 2022 St. Louis Dealmakers Conference. Smart Business Dealmakers was created by the Smart Business Dealmakers Institute to serve as a resource for news, insights, and networking for...
Immunophotonics Awarded $2.4 Million SBIR Grant by the National Cancer Institute
Immunophotonics, Inc. was recently awarded a Small Business Innovative Research (SBIR) Phase I and Phase II Fast-Track grant for research regarding use of the company’s lead drug candidate, IP-001 by the NIH’s National Cancer Institute. This research will be conducted by Immunophotonics Inc and its collaborators at the University of Louisville Division of Surgical Oncology....
Wei R. Chen B Cell Paper Publication
Hot off the press: Another part of the mechanism of action of how IP-001 (GC) remodels the tumor microenvironment revealed! Check out this key publication to understand how tumor ablation paired with IP-001 influences B cell activation, its role in extending survival, and the favorable clinical implications. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8692917/pdf/thnov12p0639.pdf
SPIE Photonics West and BiOS Expo, Jan 22 – 27 in San Francisco
If you are interested in the interface between cancer immunotherapy innovation and photonics, then catch up with Tomas Hode, PhD, co-founder, CIO, and President of Immunophotonics, Inc. in person at SPIE, the international society for optics and photonics conferences #PhotonicsWest / #BiOS 2022 Jan 22 – 27 in San Francisco. He and Lu Alleruzzo (CEO)...
USPTO Issues Patent Covering Immunophotonics’ Lead Drug Candidate, IP-001
Immunophotonics is pleased to announce that the United States Patent and Trademark has issued U.S. Patent No. 11111316, which covers the composition of matter of its proprietary synthetic biopolymer, IP-001. Immunophotonics is currently conducting clinical studies for the use of IP-001 to treat various metastatic cancer indications and is exploring other applications of the drug...
Immunophotonics Switzerland (IPS Biopharma) Invited to Present at Swiss Biotech Day
Immunophotonics Switzerland (IPS Biopharma) will be presenting at the Swiss Biotech Day in the Emerging Company session hosted by BB Pureos Bioventures. The Swiss Biotech Day is one of the premier annual biotechnology conferences in Europe, featuring panel discussions and presentations from key opinion leaders in the biotech industry. Immunophotonics’ presentation will take place on...
Immunophotonics Publishes Follow-up Feature in Nature Biopharma Dealmakers
Immunophotonics is excited to be featured in the September 2021 oncology issue of Nature Biopharma Dealmakers. Read what Dr. Markus Joerger and Dr. David Anderson, distinguished clinical and scientific experts, have to say about how Immunophotonics is pioneering the growing field of Interventional Immuno-Oncology (IIO) with its lead drug candidate, IP-001. To read the article,...
Immunophotonics Featured in Nature Biopharma Dealmakers
Immunophotonics has been featured in the March oncology issue of Nature Biopharma Dealmakers (https://www.nature.com/articles/d43747-021-00020-2 ). Nature selects only a handful of companies for such articles. The article highlights our uniqueness in pioneering Interventional Immuno-Oncology and conveys some of our recent findings.
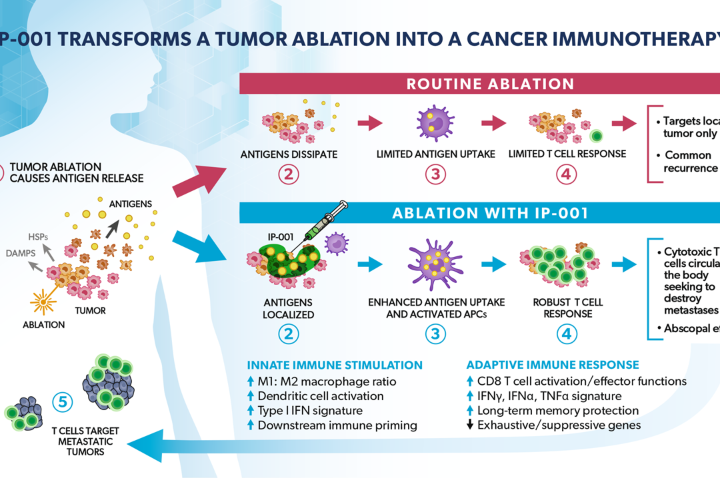
Study Shows IP-001 to Amplify Tumoricidal and Immune Effect of Cancer Ablation Therapies
A paper recently published in Cells has described how Immunophotonics’ immune-stimulating drug IP-001 was shown to be effective against metastatic tumors when combined with different forms tumor ablation. In this review, the authors briefly discuss the current applications of local ablation for cancer treatment and the effects of IP-001 in combination with other ablation therapies,...